
By R. Gary Raham
Of course you remember it — that giant chart on the wall of your first real chemistry class. The Periodic Table of the Elements. It looks rather like a checkerboard run amok, with large letters in the middle of each square, and numbers hiding out in the corners. In truth, it is a masterwork of discovery and prediction that you may not have totally appreciated — especially if chemistry class happened to fall just before lunch. If it’s any consolation, the man who put it together, Dmitri Ivanovich Mendeleev (1834-1907), was not that great of a student in high school either.
Mendeleev’s mother believed in him, though. That’s important when you’re the 17th child. Mendeleev enrolled in college and graduated at the top of his 1855 class. He became fascinated by the chemistry of matter. Like others before him, he noticed that certain patterns arose among the 63 known elements. Certain chemical properties repeated, and the repetition had something to do with the differing weights of the various elements.
In the mid 19th century the atomic theory of matter was new. John Dalton had proposed in 1808 that the basic elements were each composed of unique particles called atoms. The atoms of each element had different masses, with hydrogen being the lightest element. Measuring the weights of the various elements precisely became an important step in recognizing patterns that arose as the elements combined with each other to form compounds.
Mendeleev was not a wealthy man. He taught middle school classes while he was working on his master’s degree, and he served as a tutor and lecturer, and he was a popular writer at various times. At first, he specialized in organic chemistry (the chemistry of carbon compounds), and he wrote a handbook that won the Demidov prize in 1862 that provided some money and recognition. He earned his Ph.D. in chemistry in 1865 and became a professor at the University of St. Petersburg.
At some point he had to teach a class in inorganic chemistry. To prepare for teaching an unfamiliar subject, he made cards for each element outlining their chemical properties and began ordering those cards by the atomic weights of the elements. He noticed that an element’s valence — its capacity to combine in a fixed way with other elements — varied in the repeating pattern of 1234321. When arranged in rows of ascending weight, elements group in columns so that the elements in a given column display similar chemical properties.
What made Mendeleev’s work stand out from other chemists who had also seen repeating patterns was that to make his rows and columns work, Mendeleev’s had to insert gaps within the charts. Those gaps, he predicted, represented missing elements that no one had yet discovered. His predictions came true — several of them during his lifetime: gallium, scandium and germanium. Scientists now know that there are 92 naturally occurring elements (with uranium the last in the sequence), but other elements are still being discovered and placed on the table. Today, scientists have verified 118 elements — the last one named Oganesson after an 85-year-old chemist still trying to make additional elements. Elements beyond uranium are short-lived and decay quickly into lighter elements.
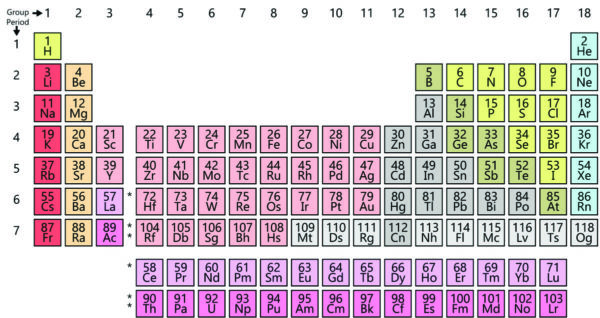
Mendeleev found this concise way of summarizing our chemical universe without the benefit of knowing the details of atomic structure. As those details unfolded, they explained why elements show repeating behavior. Most of the weight of an atom resides in its nucleus, a mixture of positively charged protons and uncharged neutrons. But elements carry no overall electric charge. The positive charge of protons is neutralized by tiny, negatively charged electrons that orbit the nucleus in layers (called shells). Only so many electrons can exist within a shell. When shells are complete, atoms tend to be aloof — chemically unreactive. The number of “left over” electrons in outer shells makes elements reactive in particular ways, which explains the observed periodicity in chemical behavior. The added heft of bigger atoms helps explain differences in how elements react and combine.
The excitement of scientific discovery comes from these insights resulting in a Grand Synthesis that unites a broad spectrum of what’s observed in nature into a unifying pattern. Physicists have Newton who unraveled the relationships of force and motion. Biologists honor Darwin for showing how natural selection leads to evolution over time. Chemists such as Peter Atkins revere Mendeleev. Atkins described the Periodic Table as “arguably the most important concept in chemistry.”
So, if your son or daughter comes home grumbling about having to study some “dumb old Periodic Table,” you are armed with information to adjust their attitudes. Delay the wonders of chemistry until after lunch, however, when you have a better shot at commanding their full attention.
Support Northern Colorado Journalism
Show your support for North Forty News by helping us produce more content. It's a kind and simple gesture that will help us continue to bring more content to you.
BONUS - Donors get a link in their receipt to sign up for our once-per-week instant text messaging alert. Get your e-copy of North Forty News the moment it is released!
Click to Donate
