Bio Bites
By R. Gary Raham
Ninety-eight percent of the known universe consists of the elements hydrogen and helium. Just hot gas. Go figure. But 25% of what’s left is carbon—an element amazingly promiscuous in forming bonds with a host of other elements. A book by Robert M. Hazen called Symphony in C reminded me of this fact and many others. Hazen is executive director of the Deep Carbon Observatory (https://deepcarbon.net/) and Professor of Earth Science at George Mason University. Hazen obviously knows something about music as well. He uses the metaphor of a symphony—an elaborate musical composition with typically four movements—to expound about “carbon and the evolution of (almost) everything.”
Carbon is the element of life. Even biologists like me without a strong compassion for the intricacies of chemistry know that. Living things are essentially bags of water with fancy carbohydrate, protein, fat, and nucleic acid carbon compounds floating around doing all the things that living things do: eat, grow, pass gas, and reproduce, for example. But carbon is much more. Crush it, and it becomes diamond. Crystalize carbon into hexagonal sheets to form graphite, and you can write with it, lubricate with it or make electrodes, batteries, and solar panels. One would think by now we clever humans would know all there is to know about carbon, but not so. Recently, chemists at Oxford University and IBM created a new stable form of carbon: a cyclocarbon.
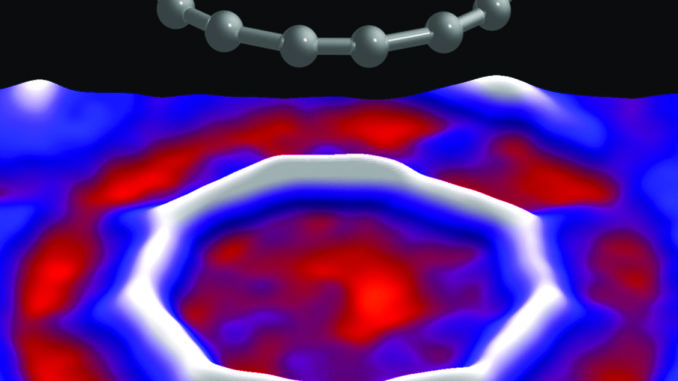
A cyclocarbon is a ring of 18 carbon atoms. To make it, the chemists started with a carbon-oxygen compound (C24O6) and severely cooled it after depositing it on a salt (NaCl) and copper (Cu) surface. This process squeezed out carbon monoxide molecules, leaving a ring of carbons connected by alternating single and triple electron bonds. (https://chemistryhall.com/cyclic-carbon-allotrope-cyclocarbon/). Visually, as revealed by an atomic force microscope, cyclocarbons look a bit like tiny bullseyes or friendship bracelets.
What kind of mischief can we create using cyclocarbons?
A writer for Popular Mechanics magazine, John Wenz, speculated that cyclocarbons might be used to create neural networks for artificial intelligences. (https://www.popularmechanics.com/technology/robots/a28705498/super-material-ai-chips/). One of the reasons for this speculation is that cyclocarbons may be able to conduct electrons at relativistic speeds, allowing for rapid transmission of impulses. Winz said, “Most ‘neural networks’ today are simulations of physical neural networks relying on a series of algorithms. But the researchers (using cyclocarbons) think they could use it to physically model neurons—an idea that stretches back to the beginnings of artificial intelligence research.”
Back in the 1980s chemists found another usual class of carbon compounds called fullerines or “buckyballs,” so named because the carbon atoms formed a structure not unlike the architectural domes created by Buckminster Fuller. Fullerines spurred research in many areas. Buckyball compounds can be used to control allergies, fight deterioration of motor function due to multiple sclerosis, to produce inexpensive solar cells, store hydrogen for fuel cells, and more.
Just between you and me—one carbon medley speaking to another, so to speak—I suspect we will find amazing things to do with cyclocarbons. What do you think?
Support Northern Colorado Journalism
Show your support for North Forty News by helping us produce more content. It's a kind and simple gesture that will help us continue to bring more content to you.
BONUS - Donors get a link in their receipt to sign up for our once-per-week instant text messaging alert. Get your e-copy of North Forty News the moment it is released!
Click to Donate